EARLY TRIASSIC AFTERMATH, SECTION 2
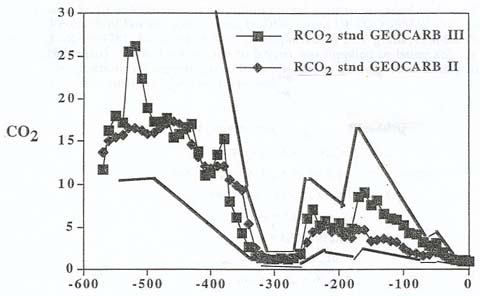 |
| The relative amount
of carbon dioxide in Earth's atmosphere over time, compared to that of the present. The data
from two studies is presented, the earlier data set represented
by squares, the most recent estimates by diamonds. The data sets
are enclosed by an upper and lower line (with jaggies), indicating
the possible margin of error. Note the abrupt carbon dioxide
increase at about 250 million years ago, the time of the end-Permian.
Another increase occurs about 180 million years ago: Could this
be related to the Early Toarcian event? (Berner and Kothavala,
2001) |
As the oxygen content of the atmosphere
was plummeting, the level of carbon dioxide in the atmosphere
increased significantly, and was perhaps five times that of the
atmosphere today. One way we know this is through the examination
of fossil leaves. The number of the leaf pores (stomata) through
which plants obtain gases from the atmosphere (a number called
the stomatal index) dropped, reflecting the higher atmospheric
concentrations of carbon dioxide (Retallack, 2001). High levels
of this greenhouse gas would have significantly warmed the planet,
and the rapid appearance of red beds in many previously temperate
areas, including in the Sverdrup Basin, seems to confirm this
global warming (see map of coal measure/red bed succession, above).
In addition to these dramatic changes
in Earth's climate, the ocean's chemistry seems to have shifted
enormously. During the last stage of the Permian, deep ocean waters
had become anoxic and sulfidic (containing high levels of sulfide)
as indicated by the presence of black shales and distinctive,
minute, framboidal (raspberry-like) grains of pyrite (Nielsen
and Shen, 2004).
(Framboid grain size is an important
indicator of oceanic oxygenation. In well-oxygenated waters, framboid
grains tend to be larger; in anoxic environments they are considerably
smaller. This may be the consequence of the depth at which they
are formed. Where deep waters are anoxic, framboids form in the
water column above the seafloor, and quickly drop into the seafloor
muds before they reach an appreciable size. If framboid formation
is confined to the anoxic seafloor sediments by oxygenated deep
water, however, they have the opportunity to grow to greater sizes:
Wilkin, 1996; Wilkin, 1997; Wignall and Newton, 1998; Nielsen
and Shen, 2004).
A million years after the end of the
Permian, even the ocean's surface waters throughout the world
were oxygen-poor (Map from Wignall and Twitchett, 2002):
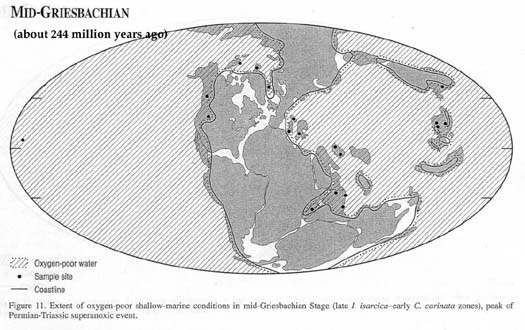
and it took several more millions of
years before this ocean surface dysoxia began to dissipate (Map
from Wignall and Twitchett, 2002):
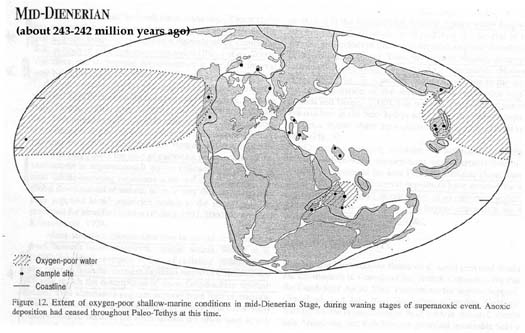
Anoxia lasted longer in the deep ocean
(Isozaki, 1994; Isozaki, 1997), apparently ending first in the
equatorial Tethys, then the Neo-Tethys (the ocean being created
as the Cimmerian continent moved away from Gondwana), and finally
in Panthalassa itself (Wignall and Twitchett, 2002). Early in
the Triassic, in all but the shallowest waters, Panthalassa was
"truly euxinic" (Wignall and Twitchett, 2002).
The term "euxinic" means that
the seawater was not merely anoxic, but that it was also sulfidic:
containing large quantities of sulfide. The sulfide was the result
of the activity of the sulfate-reducers, which strip the oxygen
from the sulfate and dump hydrogen sulfide as a waste product.
(Hydrogen sulfide is a toxic gas, and its presence in latest Permian
and Triassic seawater must have been responsible for considerable
mortality among marine organisms.) The increase of iron pyrite
(iron sulfide) in the sediments was also an indication of the
activity of sulfate-reducers. That euxinic conditions prevailed
for millions of years seems attributable to the presence of the
sulfate-reducers well into the water column, and not merely buried
in bottom sediments.
The Black Sea
The word euxinic comes from the old Roman
(and ultimately from the Greek) name for the Black Sea (the Romans
called it the Euxine Sea, Pontus Euxinus), that body of water
almost completely enclosed by Turkey, Bulgaria, Romania, Russia
and some former Soviet republics. The Black Sea derives its name
from its dark waters: unlike the Mediterranean, where visibility
extends down to a depth of about 30 meters (about 100 feet), visibility
reaches only as far as about 5 meters (16 feet) in the Black Sea.
 The Black Sea, from space. Its only outlet is virtually invisible,
to the lower left, where it flows through the Bogazi (Bosporus),
past the city of Istanbul, to the Sea of Monmara (pale blue),
and then into the Aegean (lower left) and eventually the Mediterrnean
(not seen). The Black Sea is about 1200 km (750miles) at its widest.
(NASA photo, from: en.wikipedia.org/upload/thumb/a/ae/400px-Black-sea-nasa.jpg)
The Black Sea, from space. Its only outlet is virtually invisible,
to the lower left, where it flows through the Bogazi (Bosporus),
past the city of Istanbul, to the Sea of Monmara (pale blue),
and then into the Aegean (lower left) and eventually the Mediterrnean
(not seen). The Black Sea is about 1200 km (750miles) at its widest.
(NASA photo, from: en.wikipedia.org/upload/thumb/a/ae/400px-Black-sea-nasa.jpg)
The Black Sea is black (actually, it
simply becomes darker at a shallower depth than other large bodies
of water) because it is darkened by the presence of "gelbstoff,"
a German word literally meaning, 'yellow stuff.' This is dissolved
organic matter, of the sort produced when autumn leaves are soaked
in water, coloring it yellow, and making it dark at a relatively
shallow depth. A recent intruder, a comb jelly (Mnemiopsis. Comb
jellies, scientifically known as ctenophores -- pronounced "teen-a-fores"
-- employ comb-like bands of fused cilia for locomotion, hence
the name) also dumps a lot of mucus into the water. This mucus
catches particulate matter that would otherwise sink to greater
depths and and keeps it close to the surface, thus contributing
to the water's present dark color, though the name "Black
Sea" is ancient (Sorokin, 2002).
Although we know too little at present
to be able to describe what the anoxic ocean of the Early Triassic
may have been like, we are learning more, based mostly on our
increasing understanding of the Black Sea. The Black Sea is unique
among the larger bodies of water on the planet, being anoxic in
its deeper waters. It is, in fact, the world's largest anaerobic
basin (Egorov, 2003). The more shallow waters are oxygenated,
but below 80 to 100 meters (yards) depth the waters are at first
dysoxic and then fully anoxic.
The Black Sea is also the world's largest
meromictic body of water. The term meromictic refers to the limited
vertical circulation of the sea. Vertical circulation is limited
because the Black Sea essentially has two layers: an upper layer
about 100 to 200 meters (yards) thick that sits on top of the
rest of the sea's water. These two layers are quite distinctive
in their density, with the lower layer being considerably more
dense than the upper layer. The density difference is due to the
great salt content difference between the layers.
The upper layer is less salty, in part,
because the Black Sea receives fresh water from the great rivers
that drain eastern Europe and southern Russia: the Danube, the
Dnestr, the Dnepr, the Don. Being less salty, the fresh water
is less dense, and remains on the sea surface. But the Black Sea
also receives much of its water from the eastern Mediterranean
via the Istanbul Bogazi (···the g has a small
u over it···), or Bosporus. Eastern Mediterranean
water is very salty because it receives little freshwater input
in relation to its high rate of evaporation. The high salinity
of the water makes it quite dense. Once this water flows past
Turkey's largest city, Istanbul, into the Black Sea, it quickly
drops down to the deeper part of the Black Sea basin. (A return
flow through the Bogazi -- the Black Sea's only outlet -- carries
Black Sea surface water into the eastern Mediterranean.) As a
result, the lower layer of the sea is roughly 1/3 more saline
(salty) than the upper layer. It is this vast density contrast
which prevents free circulation between the two marine layers.
This density and salinity difference
did not always exist in the Black Sea. In fact, it did not exist
as recently as about 10,000 years ago. At that time, the Earth
was just emerging from its most recent ice age. Sea level was
much lower, because much of the planet's water was tied up in
the great continental ice sheets. Consequently there was no seawater
input to the Black Sea from the Mediterranean: the Bogazi (or
Bosporus) sill (a ridge, now underwater) is relatively shallow,
and lowered sea level effectively converted it into a dam (Sorokin,
2002).
With the melting of the great ice sheets,
global sea level rose. Water filled the Mediterranean sufficiently
that it began to flow over the sill into the Black Sea. Over the
course of the next several thousands of years, the bottom water
became much more saline, and the sea became stratified. The boundary
between the distinct densities (known as the pycnocline) rose
ever higher as the salty eastern Mediterranean water filled the
bottom of the sea. The depth of the boundary varies from place
to place, due to factors like river input, precipitation, and
seasonal temperature changes, but it ranges from 100 to 200 meters
(yards) in depth, though usually it is between about 125 and 175
meters. The boundary, moreover, is not sudden, but represents
a rapid but gradual change from one density to another over a
distance of several tens of meters (Sorokin, 2002).
In addition, the pycnocline also marks
the halocline (the rapid change in salinity between lower and
upper water layers) and a chemocline (where water chemistry significantly
changes). Above the pycnocline, Black Sea water is oxigenated,
both from river input and surface turbulence. Below, it is anoxic,
because the surface layer and deeper waters largely fail to mix.
At the boundary, which is about 50 meters
thick, the oxygen in the surface layer gradually disappears from
the water column, and the hydrogen sulfide that is characteristic
of the deeper layer appears. The boundary is a place of intense
microbial activity. It hosts a flourishing community of microorganisms,
but many are found here in numbers not known elsewhere. Aerobic
organisms use the oxygen of the upper layer to consume the gases
methane and hydrogen sulfide that are produced below.
Also at the interface between the overlying
oxic waters and the anoxic waters below are green sulfur bacteria
(Chlorobiaceae), which are strict anaerobic organisms. These microorganisms
rely on (and also oxidize) a supply of hydrogen sulfide, produced
by the sulfate-reducing anaerobes that inhabit the water column
below them (Pimenov, 2003; Sinninghe Damsté, 2003). Perhaps
most surprisingly, they are photosynthetic, and use the minute
amounts of light that penetrate to the 80 to 100 meter (yard)
depths which they inhabit (the upper part of the ocean, into which
light can reach, is known as the photic zone). At such depths,
light is so faint that the green sulfur bacteria's chlorophyll
molecules on average absorb only a single photon (light particle)
every eight hours (Overmann, 2003). The photosynthesis process
mediated by these organisms is also anoxygenic, meaning that it
does not produce oxygen, as does most photosynthesis.
Green sulfur bacteria employ specific
pigments that allow them to conduct photosynthesis (the aromatic
carotenoids isorenieratene and chlorobactene). These pigments
rapidly degrade after the death of the hosting organism, but their
derivative organic chemicals are much longer-lasting, and can
be used as biomarkers (biological indicators) to establish the
former presence of green sulfur bacteria in ancient oceans. In
fact, these molecular fossils have been found in rocks as old
as 450 million years, and indicate that "Black Sea"
conditions have been common in Earth's history (Sinninghe Damsté,
2003). And, more specifically, green sulfur bacteria biomarkers
have now (January 2005 report) been found in the sedimentary rocks
which define the Permian-Triassic boundary. These biomarkers proclaim
that, like today's Black Sea, the oceans of the end-Permian and
Early Triassic were euxinic, not only in their darkest depths,
but up to where light could penetrate (about 80-100 meters/yards;
Grice, 2005).
At the Black Sea's oxic/anoxic boundary,
anaerobic organisms such as sulfate-reducers consume the rain
of organic material falling from above, producing hydrogen sulfide,
while anaerobic methanotrophs reduce the concentration of methane.
Much of the methane is generated far below, in the seafloor sediments,
but most is consumed at the oxic-anoxic boundary, by the aerobic
methanotrophs. A small amount [a little over 1%] escapes through
the surface layer and into the atmosphere (Hunt, 1974). But unlike
along the Namibian coast, the hydrogen sulfide does not escape
its confinement, and remains below about 125 meters. This is partly
because the gas is relatively heavy, but mostly because it is
consumed by the sulfide-oxidizing organisms of the aerobic zone.
Ammonia (NH¸3) also increases with depth, along with acidity
(Sorokin, 2002).
Despite aerobic organisms being confined
to the surface layer, that surface layer is deep enough to support
a Black Sea fishing industry. In the shallower parts of the sea,
aerobic invertebrates also flourish. Nonetheless, with about 80%
of the Black Sea's water being in the anoxic zone, large, aerobic
organisms are not found in the depths. Instead, the deep is the
realm of anaerobic, single-celled organisms, giving rise to the
description of the Black Sea as a bacterial sea. Undoubtedly this
name derives from the period before the discovery of archaea;
a more descriptive term now would be microbial sea, because both
bacteria and archaea are microbes.
The surface water of the Black Sea, despite
being oxygenated, contains two to three times more methane than
ocean surface waters elsewhere (Egorov, 2003). This is likely
to be the consequence of the numerous methane seeps found in the
muds on its continental margins, many of which are in the delta
regions of the great inflowing rivers. As the rivers bring down
large quantities of organic debris, they furnish methanogens with
abundant food supplies.
Interestingly, however, while the distribution
of methane in the deeper anoxic waters is fairly uniform (Egorov,
2003: Ivanov, 2003), the amount of methane in deep sea sediments
is often less than in the immediately overlying waters. In addition,
the sediment methane content frequently decreases with depth (Egorov,
2003; Ivanov, 2003). This indicates that -- at least in these
areas -- the sediment cannot be the sole source of the methane
found in the water column.
Elsewhere the sediment methane does increase
with depth (Egorov, 2003; Ivanov, 2003). Presumably these are
source areas where methane is actively being produced by the activity
of methanogens in the sediment. A recent expedition found a sediment
surface which was dimpled with small craters, from which methane
bubbles were being produced. On one occasion, strings of bubbles
were seen rising into the water. The sediment surface was black
(possibly from sulfides, which are often black), and was covered
with mats of microbes. But a second examination of the area a
year later failed to show the same features: there were no chains
of bubbles, no sediment dimpling, no microbial mats, and no black
color to the sediment surface, which had become brown (Egorov,
2003). Obviously, some methane seeps are not constant in their
production.
In other methane producing areas, the
volume of methane must be considerably greater than that issuing
from these seeps. Mud volcanoes up to a kilometer or two in diameter
punctuate the Black Sea floor. Methane-bearing fluids escape along
faults and through chimneys as much as 500 meters (yards) in diameter.
Abundant calcium carbonate crusts (in the mineral form known as
aragonite) -- remember those "anomalous carbonates"
noted by Knoll (1996) from the end-Permian seafloor? -- are found
with the microbial mats associated with these seafloor features
(Ivanov and Stadnitskaia, 2003; Ivanov, 2003; Yu, 2003). Seismic
images show the chimneys to be the same type of "wipeout"
(sonar transparent) features identified elsewhere (Wood, 2002;
Hornbach, 2004).
The current condition of the Black Sea
undoubtedly reflects its similar condition in earlier interglacial
episodes. When great ice sheets sprawled across the land, sea
level was lower and the Black Sea fresher and better mixed and
oxygenated. As the ice sheets melted, the Black Sea became stratified,
and anoxic in its depths. Presumably euxinic (sulfidic) conditions
were a regular though intermittent condition of the Black Sea
during the course of the 2.4 million years of the Ice Age, as
the growth and melting of continental ice sheets lowered and raised
global sea level. When ice sheets spread across the continents,
the Black Sea, isolated from the Mediterramean, would have been
fresh; when global sea level was high during interglacial periods,
the Black Sea would have been euxinic.
For about 10 million years at the beginning
of the Triassic Period, features similar to those of the Black
Sea characterized the ocean. Panthalassa and the Tethys were stratified,
anoxic and euxinic at depth, but their surface waters were probably
at least locally and intermittently oxygenated. Certainly the
mouths of large rivers would have carried oxygenated river water
into the nearby surface ocean, and the survival of fish and other
large aerobic organisms -- crustaceans, molluscs, echinoderms,
marine worms, anemones and so on -- shows that marine anoxia was
not universal.
Globally higher levels of carbon dioxide
would also have made the surface ocean more acidic. Nonetheless,
the surface ocean could have been quite fertile, from the enhanced
weathering of the land by acid rain, the addition of volcanic
dust, and the anoxia-produced buildup of marine phosphate. (On
the other hand, oceanic anoxia could have permitted the concentration
of another essential nutrient, "fixed" nitrogen [NH¸4^+,
NO¸2^, and NO¸3^, that is, the ammonium ion, nitrite,
and nitrate, respectively], to be seriously reduced. This because
certain bacteria [of the bacterial order Planctomycetales]
have the ability to oxidize ammonium anaerobically. These organisms
apparently are common in anoxic basins and in areas of strong
upwelling, as along the Namibian coast [Kuypers, 2005].) But oceanic
fertility does not necessarily translate into more favorable environmental
conditions for larger aerobic organisms. The increase in essential
nutrients could have resulted in surface algal blooms that deplete
oxygen in the water below, and would explain why transient anoxia
seems to have affected even shallow waters in the early Triassic
(Wignall and Twitchett, 2002). Large aerobic organisms would have
faced severe environmental challenges wherever they lived.
Exactly how good a model the Black Sea
is for the great ancient oceans of Pangea -- Panthalassa and the
Tethys -- after the end-Permian catastrophe, will have to be determined
by further research. There are clearly ways in which the Black
Sea would not have resembled the oceans of the Permian: the Black
Sea receives much of its water from the highly saline (and therefore
quite dense) eastern Mediterranean, which establishes a strong
density contrast -- with little mixing and therefore considerable
isolation -- between its bottom waters and its surface waters.
In addition, although it does receive a large (and oxygenated)
inflow from major European rivers, this input is small compared
to the total volume of the Black Sea itself, allowing for oxygen
depletion over thousands of years. It is, in fact, this imbalance
between fresh water inflow and total water volume which has caused
Black Sea deep water anoxia (Gross, 1990, p. 256).
In some ways, however, the Black Sea
may be quite a good model for the end-PermianTriassic oceans.
Undoubtedly many species of microorganisms from the end of the
Permian and the Early Triassic have not survived to the present
day. Yet organisms performing similar ecological functions, and
living in similar habitats, are likely to have been present 250
million years ago. In fact, new research (reported January 2005)
"provides unequivocal evidence [regarding] photic [lighted]
zone conditions" at that time (Grice, 2005). The evidence
includes biomarkers for green sulfur bacteria, as well as other
indicators of at least intermittently euxinic photic zone conditions.
The composition of iron residues from Early Triassic rocks are
"comparable to values reported from the modern euxinic Black
Sea," and sulfur isotopes in those rocks "are consistent
with euxinic conditions as found in the modern Black Sea"
(Grice, 2005).
In addition, the general features of the Black Sea -- its oxic
surface waters, down to 80 or 100 meters; its anoxic bottom waters;
the lack of fish and other aerobic organisms in the deeper waters;
the domination of bottom waters and the seafloor sediments by
archaea and anaerobic bacteria, creating a kind of anaerobic microbial
soup -- and those of the Early Triassic are likely to prove very
similar. (The coming methane catastrophe is likely to produce
marine conditions more similar to those of the Early Triassic
than those of today's Black Sea, because of the expected high
levels of atmospheric and oceanic carbon dioxide.)
The unusual conditions of Early Triassic,
in the aftermath of the Permian extinction, are in contrast to
those following the end-Cretaceous catastrophe that did in the
dinosaurs and so many other organisms. Although in many areas
of the planet recovery after the K-T impact was protracted, in
others it was quite rapid. In much of North America, which many
scientists believe suffered the worst impact-related effects because
it was in the path of the molten rock droplets which splashed
from the Chicxulub Crater, floral diversity remained low for millions
of years after the impact (Johnson and Ellis, 2002).
But in other North American areas, recovery
was astonishingly fast. Within one and a half million years, a
thriving rainforest had appeared in then-tropical Colorado. Fossils
from Castle Rock, not far from Denver, appear to come from a forest
floor. These fossils reveal the forest had a diversity of species
greater than that found in today's Brazilian Amazon, as well as
a considerable number of new species and large tree trunks and
leaves. Many of the leaves exhibit elongated tips, known as drip
tips, which are commonly found in rainforest trees to facilitate
the shedding of water (Johnson and Ellis, 2002).
In ten million years -- the same amount
of time during which the effects of the Permian devastation continued
to be acute -- the surviving mammals from the Cretaceous had already
evolved into many of today's mammalian groups. By about 50 million
years ago, exquisitely preserved fossils from Messel, Germany
reveal a diversity of bats and the diminutive ancestors of modern
horses, among the remains of many other organisms (Schaal and
Ziegler, 1992).
There was no such rapid recovery after
the end of the Permian. Life had been dealt a stunning blow, and
recuperation was enormously protracted. Even more than the Permian
extinction itself, it is the length of this recovery which demands
explanation. Mass extinctions, whatever their particular causes,
seem to take place and to be over quickly, at least on a geological
time scale. In their aftermath, other organisms rapidly occupy
vacant ecological niches from those which went extinct.
Not so with the Permian extinction. The
ten million years it took for partial recovery, and the twenty
it took for a full recovery -- understanding that here full recovery
means a flourishing biota, even though its constituent organisms
may have been quite different from those which came before --
render the Triassic recovery unique. The continuing biotic malaise
to and perhaps through the Middle of the Triassic demands explanation,
and that explanation must be related to the nature of the Permian
extinction itself.
Explaining the Gaps
The Chert Gap:
The causes of the Early Triassic Chert
Gap seem quite simple: anoxia, ocean warming, and ocean acidification.
Sponges, being animals, need oxygen. One basic effect of the Traps
volcanism coupled with the release of substantial amounts of hydrate
methane was to slow or stop normal thermohaline circulation, changing
ocean bottom water from oxygenated to anoxic. Without oxygen,
the sponges died. Those hyalosponges whose activities had resulted
in the Permian Chert Event also required the water in which they
lived to be quite cold. With altered oceanic circulation and warming,
hyalosponge habitats were denied that cold.
In addition, both hyalosponges and radiolarians,
another major producer of the siliceous ooze that hardens over
time into chert, would have been seriously and adversely affected
by oceanic acidification. These organisms obtain the silica they
need for their skeletons from that which is dissolved in seawater.
This silica, which is typically in short supply, is more readily
dissolved in alkaline water. As ocean chemistry changed and ocean
water became more acidic, even less silica would have been available.
Until cold, oxygenated, and more alkaline conditions returned,
therefore, chert production and deposition would have ceased.
The Reef Gap:
Reef ecosystems are notoriously vulnerable
to what may seem to be very small changes in temperature. A few
degrees warmer water can devastate a reef ecosystem, and, in recent
years, many have been succumbed to just such a cause. When one
considers the conditions that ordinarily prevail in reefs, this
is not hard to understand. Reefs normally enjoy highly stable
conditions. Water temperatures remain steady over long time periods,
varying only slightly from season to season. The oxygen content
of the water, its salinity, its slight alkalinity (normal ocean
pH is 8.1), its clarity, and nutrient supply are generally quite
constant.
Every one of these conditions would have
been affected by protracted volcanism and hydrate methane release.
Water temperatures would have risen. The relative acidity (pH)
would have been changed by acid rain. Seawater would have become
dysoxic, and coral reef organisms require oxygen. The oceanic
nutrient supply would have been enhanced by episodic volcanic
ashfalls and the continuous weathering of continental rock by
acid rain, but the upwelling of nutrients would have been much
diminished in a stagnant ocean.
But even a plenitude of nutrients is
not necessarily a good thing. An excess can cause surface water
algal blooms which deny oxygen to the water below. The introduction
of "biodegradable" detergents in the 1960's brought
disaster to Lake Erie, the shallowest of the Great Lakes, because
the biodegradable phosphates being dumped into the lake caused
unprecedented algal blooms. At the eastern end of the lake was
an enormous (200 square mile), two-foot thick scud of algae, and
the shores of the lake were covered with the rotting corpses of
suffocated fish.
Though corals were not major constituents
of the Permian reefs, the corals themselves were particularly
hard hit by the extinction event. Two major groups of corals,
the rugose corals and the tabulate corals, disappeared forever.
In part, this may reflect the need of corals to build calcium
carbonate skeletons, a need that would have been difficult to
fulfill as ocean waters became more acidic. In addition, many
corals contain photosynthetic algal symbionts. During periods
of reef stress (as during warming), these symbionts are frequently
ejected. While such ejection may help a coral deal with stress
on a short-term basis, in the long run it deprives the coral of
a major food source. And as the photosynthesizers also contributed
oxygen, corals may have been deprived of an oxygen source just
as they found themselves in increasingly dysoxic waters.
There are many ways to kill a reef, and
indeed, many of them were present in the end-Permian catastrophe.
Moreover, the warmth, increased acidity, and dysoxia are all conditions
which would have been sustained for long periods of time.
The Coal Gap:
The coal gap is more difficult to explain.
One thing that does have a considerable impact on terrestrial
vegetation, however, is acid rain. This is well known from the
experience with acid rain in the United States and other industrial
nations. Industrial emissions include numerous gases (sulfur dioxide,
nitrogen oxides, and carbon dioxide, which are also present in
volcanic emissions) which chemically react with atmospheric water
to produce acid rain. This rain typically falls far downwind of
the emitting plants, but it severely compromises the viability
of both terrestrial and freshwater organisms.
Protracted episodes of severe acidity,
triggered on one hand by pulses of hydrate methane release from
seafloor slumps, and on the other by pulses of volcanic eruption,
over a period of tens to hundreds of thousands of years, coupled
with increased precipitation, would have been quite sufficient
to cause the extinction of many species of vegetation, including
those whose peaty and humified remains became coal. More generally,
mycorrhizal fungi, the root symbionts which provide most terrestrial
plants with soil nutrients, may have been particularly vulnerable
to this altered environment.
Those plants compromised by this assault
would have been further damaged by unfamiliar environmental conditions,
including increased warmth, lower levels of atmospheric oxygen
(hypoxia), and elevated levels of carbon dioxide. Every species,
it should be remembered, is the product of long ages of honing
by the environment. As a result, each is adapted to a limited
range of environmental conditions. When conditions shift out of
that range, the organisms of that species cannot continue to exist.
Even within that range of conditions, organisms may already have
been pushed to the limits of their tolerance. When some small
additional change occurs, or a combination of small changes, the
species may no longer be able to survive. The extinction of many
peat-forming plants at the end of the Permian, and their slow
replacement during the Middle Triassic has thus been cited as
the reason for the Coal Gap (Retallack, 1996).
Additional long-term effects
The leaching of soil nutrients and the compromising of mycorrhizal
fungi by protracted acid rain would have struck the Permian terrestrial
ecosystem at its most vulnerable point. Plant life is at the base
of the terrestrial food chain, and the herbivores that depend
on those plants, as well as the carnivores that depend on those
herbivores, and the omnivores that rely on both plants and prey,
would all have suffered greatly from a loss of terrestrial vegetation.
Animals also require a certain level
of oxygen, and they would have been affected by the lowered levels
of atmospheric oxygen caused by the oxidation of methane, reduced
photosynthesis, and reduced carbon burial on the ocean floor.
It seems unlikely that they would have suffocated, because the
oxygen reduction would have been gradual over an extended period
of time, but their viability would have been impaired. When human
beings who normally live at or near sea level go to higher altitudes,
we often find that we need to adjust to the altitude. Walking
and other physical activity initially is more difficult, because
we are getting less oxygen than we are used to. Mental processes
are slowed as well, and in some cases, thought becomes muddled.
Moderate hypoxia is not a significant
problem for most otherwise healthy human beings, but it can be
for predators who depend on their speed and full mental faculties
to outrun and outwit their prey, as well as the prey animals which
require their full physical and mental faculties to escape. Although
some scientists (for example, Retallack, 2003) have suggested
that disorders akin to "mountain sickness" -- including
nausea, headache and pulmonary edema -- could have plagued Early
Triassic terrestrial animals, lowered atmospheric oxygen levels
need not have had such severe consequences to have affected viability.
Gradual oxygen deprivation could easily
have made more prey animals victims, or, conversely, allowed more
of them to escape from marginally disoriented predators. A key
word here is "compromised." A declining oxygen level
may not directly kill or even disable organisms, but it may compromise
them enough that their continued existence is put in jeopardy.
With the continuing decline of atmospheric oxygen over thousands
to millions of years, however, organisms that had fully adapted
to high oxygen levels that no longer existed anywhere on the planet
would have been decimated. Coupled with a generally reduced food
supply, lower oxygen levels would have threatened and taken the
lives of many organisms.
Why was recovery so protracted?
The hydrate methane release that marked the end of the Paleocene
at 55 million years ago provides us with a model of how such a
release and the recovery from that release should occur. The release
causes a carbon isotope excursion that lasts for about 10,000
years, and then the isotopic values return to about their previous
"normal" over some 100,000 to 150,000 years. This is
obviously a far shorter period -- by as much as a hundredfold
-- than the 10 million years or so that it took for the world
to recover from the events at the end of the Permian. The pulsed
nature of the Traps eruptions could have provoked hydrate methane
releases over a longer time period, but most scientists believe
that these eruptions were over within no more than a million years,
and quite possibly as few as 600,000. The triggering event --
the Traps volcanism -- was relatively short-lived; by contrast,
the ecological disruption quite protracted.
It does seem clear that there were episodic
releases of seafloor methane (or perhaps occasional episodes of
increased atmospheric methane, facilitated by the presence of
hydrogen sulfide: Kump, 2005) during the ensuing 10 million years
(Krull and Retallack, 2000; de Wit, 2002: Payne, 2004). These
additional releases, presumably the result of ongoing oceanic
warmth, would have continued to draw down marine and atmospheric
oxygen, reheated the planet, and caused further episodes of acid
rain. The recovery process would likely have been beset by a series
of reversals. Moreover, the biosphere had suffered a mighty blow,
and the consequences of severe food web breakdowns together with
drastically altered ecological and biochemical conditions would
have made recovery both difficult and prolonged.
But even numerous major submarine slides
and consequent methane releases should only have punctuated an
Early Triassic recovery. Instead, it seems that the vitality of
the global Early Triassic biosphere had been sapped, and that
life was suffering from an enduring malaise, quite apart from
its intermittent though devastating setbacks.
An Anaerobic Ocean
Such delayed recovery is surprising.
As Darwin, taking his cue from Thomas Malthus's Essay on Population
(1798), noted in Origin of Species (1859), the populations
of organisms tend to increase extremely rapidly (exponentially).
He states, "There is no exception to the general rule that
every organic thing naturally increases at so high a rate, that,
if not destroyed, the earth would soon be covered by the progeny
of a single pair." Darwin then gives several examples of
how extraordinarily fast that increase could be. His examples
include man, plants, and elephants. Darwin calculates that though
the reproductive rate for elephants is quite slow, a single pair,
if all offspring survived, could give rise to nearly nineteen
million elephants in just 740 to 750 years (Origin of Species,
1859, Chapter III).
In the course of the millions of years
that followed the end-Permian extinction, therefore, many surviving
organisms, no matter how few their numbers, ought to have occupied
all the ecological space left fully or partially vacant, undergone
quite dramatic population explosions and essentially recolonized
the planet. (This is kind of the biological version of "Nature
abhors a vacuum"!) Obviously, that did eventually happen
(else we would not be here!), but it took much longer than it
seems that it ought to have. Though some organisms recovered swiftly
-- the few ammonite species that survived, for example, had diversified
to more than 150 within about five or six million years (Stanley,
1987, p. 109-10) -- they were the exceptions. What caused the
delay for most other organisms? The question has perplexed paleontologists.
There are numerous answers, all of which
probably played a role. One is that organisms live in complex
food webs, and the recovery of those higher on the food chain,
like carnivores, depends on the recovery of those lower down --
herbivores, then the plants they eat, and so on. (The recovery
problem is even worse for parasites in the guts of the carnivores!)
The need to reconstruct complex food webs certainly played a role
in delaying recovery. Even so, by contrast, the rapid post-Cretaceous
recovery in at least some parts of the world (Johnson and Ellis,
2002) suggests that surviving organisms should have come back
more quickly.
Another answer is that the survivors of the catastrophe simply
occupied all the newly vacant, now available, ecological real
estate, and, as squatters, made it difficult for other organisms
to find places to live and make a living. Virtually all descriptions
of Earth's biota after the main pulse of the extinction indicate
that it was of low diversity and cosmopolitan. The low diversity
is easily understandable: the other guys had been killed off.
As used by paleontologists, cosmopolitan means that species were
widespread, rather than being restricted to smaller ranges. The
fossil record thus confirms that the survivors did occupy the
available real estate. But because they were not specifically
adapted to all the nooks and crannies of their wide ranges, over
time they would have been outcompeted by organisms which were
a better fit for particular ecological conditions. That such a
process would have taken millions of years is not surprising.
Still another possibility is that environmental
conditions were so altered that recovery was greatly protracted.
Certainly that was true to a significant extent. There was lots
more carbon dioxide around, both in the ocean and in the atmosphere.
There was less oxygen, probably a lot less. There was considerably
more rain, and it was more acidic. The ocean was more acidic,
and largely anoxic and euxinic. Its overturning circulation may
have been brought to a standstill. The world, and the ocean, was
considerably warmer.
But the additional rain would have increased
the rate of rock weathering, and the weathering of silicate rocks,
over long periods of time (about 100,000 years or so), draws down
excess atmospheric carbon dioxide. Recovering oceanic photosynthesizers
and land plants should also have helped draw down carbon dioxide.
As carbon dioxide levels dropped, the planet would have slowly
cooled, and thermohaline circulation gradually restored. The weathering
of limestone, and the ordinary oceanic buffering by dissolved
carbonate and bicarbonate, would have helped neutralize the acidity
of the oceans. In any case, both the additional precipitation
and the acidity of that precipitation should have been relatively
short-term occurrences: not lasting for too long after the cessation
of Siberian Traps volcanism, and the major release of seafloor
methane. Surely recovery should have been well under way within
a million or so years. But it was delayed for ten times that length.
The main engine of the delayed recovery
was likely the changes in the biological balance of the oceans.
The initial Eocene recovery after the Late Paleocene Thermal Maximum,
we should recall, was characterized by a "transition fauna"
in the oceans (Norris and Röhl, 1999). Similarly, the black
shale that characterizes the Japanese deep sea Permian-Triassic
boundary section "was probably a result of transient blooming
of anaerobic biota during the 'superanoxia'" (Isozaki, 1997b).
The characterization of this blooming as "transient,"
however, may not meet the ordinary definition of the word. Transient
does not seem to be an apt description of a condition that seems
to have lasted about ten million years. Perhaps the term anaerobic
resurgence is more appropriate, because it better portrays what
probably occurred in the Pangean oceans. Here is why.
Initially, the release of methane from
ocean sediments would have drawn down marine oxygen. Coupled with
the slowdown or shutdown of global thermohaline circulation, the
ocean would have become stratified, and, in its depths, largely
anoxic, killing off large numbers of animals (including the ecological
equivalents of today's giant larvaceans, if there were any), and
aerobic bacteria. Global warming would have limited the ability
of the ocean to hold dissolved gases of any kind, and placed serious
constraints on the cold-adapted organisms which constructed siliceous
skeletons. Increased marine acidity would have made the production
of calcareous skeletons much more bioenergetically expensive,
and, with the increased warmth, killed off the reefs. The Permian
phytoplankton, both with and without skeletons, would have been
seriously impacted by the ocean warmth, increased acidity, and
nutrient deprivation. Consequently, phytoplankton numbers would
have been quite reduced.
Into this breach moved the anaerobes,
archaeal and bacterial. They had always survived in the more dysoxic
and anoxic parts of the ocean (such as estuaries and fjord bottom
waters, and the oxygen minimum zone, the marine layer at roughly
500 meters/yards depth where the decomposition of organic debris
by aerobic bacteria results in oxygen depletion) and in ocean
sediments. Now they had the opportunity to reclaim a good portion
of the environment they had occupied in the early part of Earth's
biological history, possibly through the Proterozoic (2500 million
to 543 million years ago).
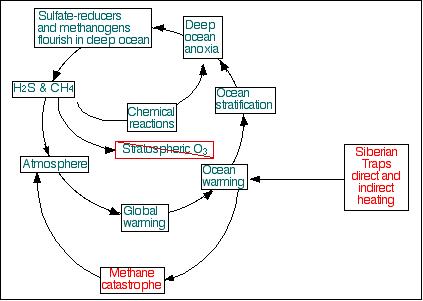 Simplified schematic diagram
of the end-Permian methane catastrophe.
Siberian Traps volcanism triggered methane catastrophe, leading
to global and oceanic warming, and ocean stratification. Once
the deep ocean became anoxic, anaerobes took over the deep ocean,
and the cycle indicated in blue-green became dominant, maintaining
altered conditions for millions of years.
Simplified schematic diagram
of the end-Permian methane catastrophe.
Siberian Traps volcanism triggered methane catastrophe, leading
to global and oceanic warming, and ocean stratification. Once
the deep ocean became anoxic, anaerobes took over the deep ocean,
and the cycle indicated in blue-green became dominant, maintaining
altered conditions for millions of years.
The anaerobes would have done this simply
by taking advantage of the new conditions of the ocean, which
was anoxic, stratified, and warm. In fact, once the deep ocean
was fully anoxic, it would have been inevitable that anaerobes
would have taken over the global ocean. Having moved back into
the larger ocean from their oxygen-restricted enclaves, their
ordinary activities of methane production and sulfate-reduction
would have helped maintain global warmth and oceanic warmth, stratification,
anoxia, and euxinia for an extended period of time. Once global
environmental conditions had been so altered, it would have been
difficult to convert the oceans back to the cooler, well-ventilated
(oxygenated), vigorously-circulating oceans that seem to have
characterized most of the Phanerozoic.
To evict the anaerobic squatters, marine
phytoplankton recovery (with an assist, perhaps, from land plants)
would have been essential. These organisms, over time, would have
dumped oxygen into the atmosphere, helping cool down the overheated
planet and restoring the ecological conditions that had prevailed
before the end-Permian environmental crisis. The process of evicting
the anaerobes from the oceans, however, would likely have been
a long one. Lasting, in this scenario, for many millions of years.
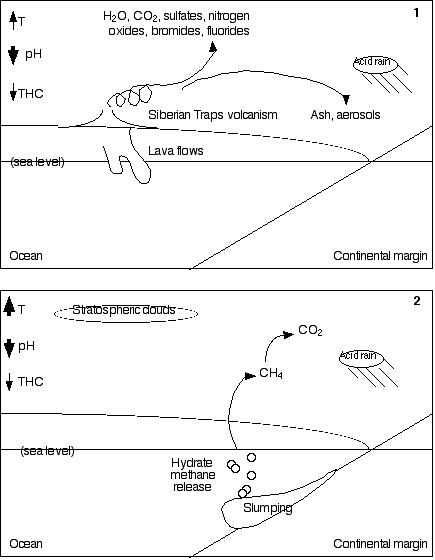
Methic world scenario. In 1, Siberian Traps volcanism
sends water vapor, carbon dioxide, sulfates, nitrogen oxides,
bromides, and fluorides into the atmosphere. The water vapor and
carbon dioxide are strong greenhouse gases; the sulfates cool
the atmosphere. Carbon dioxide, the sulfates, and the nitrogen
oxides produce acid rain (which also increases continental weathering),
and bromides and fluorides are toxic. Volcanic ash tends to cool
things down by reflecting sunlight, but it also darkens the ice
and snow in north polar regions, ultimately assisting melting
and reducing reflectivity (albedo), making the planet warmer.
Magma intrusions and lava flows release permafrost and polar continental
margin methane. (T = temperature; pH is acidity/alkalinity:
a decrease indicates greater acidity; THC is thermohaline circulation:
a decrease means that the deep oceans become warmer and less oxygenated.
Narrow arrows indicate a small increase or decrease; wide arrows,
a large one.) In 2, warming
ocean temperatures cause increased hydrate methane dissociation,
and sudden methane releases due to slumping. Increased methane
in the atmosphere not only warms the planet more rapidly (causing
ocean stratification), but also increases the cloud coverage in
Earth's stratosphere. The effect of this increased cloud coverage
is as yet unknown, because cloud dynamics are poorly understood.
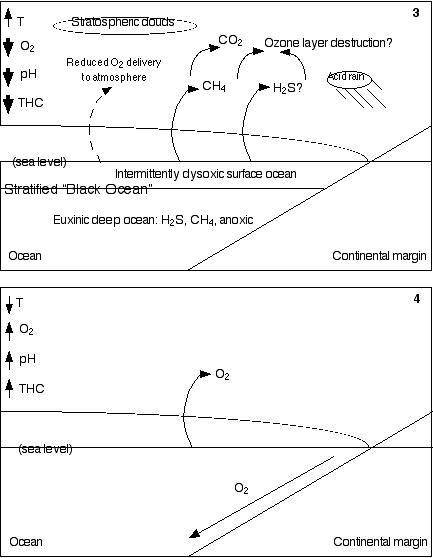
In 3,
the ocean has become stratified, so there is little interaction
between the shallow and deep ocean. It has also become largely
anoxic and anaerobic microbes have taken over in the depths. Because
of the deep ocean's high concentrations of hydrogen sulfide and
methane, resembling today's Black Sea, I have named this condition
the Black Ocean. The Black Ocean is not merely anoxic, it is also
euxinic (sulfidic), meaning that it has high concentrations of
sulfide, both hydrogen sulfide, which is toxic, and iron sulfide
(pyrite), which precipitates on the ocean floor, and is found
in black shales. The black shales (organic-rich sedimentary rocks)
would have been produced by the relative inefficiency of anaerobic
decomposition as compared to that performed by aerobes, which
permits undecomposed organic debris to accumulate in the sediments
of the seafloor. Highly disrupted "warring states" conditions
in the surface ocean presumably allowed the rise and demise of
several successive microbial "regimes." Hydrogen
sulfide and methane are intermittently released into the atmosphere,
where they can work together to destroy the ozone layer. The reduced
numbers of phytoplankton and perhaps the reduction of carbon burial
(a consequence of the deaths of aerobic organisms functionally
equivalent to today's giant larvaceans) limits oxygen delivery
to the atmosphere, resulting in lower global oxygen levels. In
4, after five to ten million years, the increased weathering
of silicate rocks begins to reduce the level of carbon dioxide
in the atmosphere, allowing the planet to cool. Photosynthetic
organisms reassert a dominance they had lost during "warring
states" conditions, but this time via the red plastid lineage
rather than the previous green plastid lineage. The gradual restoration
of thermohaline circulation carries cold, oxygenated water to
the deep ocean, and oxic conditions again prevail throughout most
of the ocean.
How would one go about obtaining evidence
for such an anaerobic resurgence?
The oceans of yesteryear, like the snows
of Francois Villon ("But where are the snows of yesteryear?"
echoed in Joseph Heller's Catch 22: where are the Snowdons of
yesteryear?), are gone forever. Their sediments, containing the
fossils from that time, are likewise gone, either back into Earth's
mantle as the ocean plates were subducted or, in rare cases, accreted
onto land. As the oldest ocean floor is only 160 to 180 million
years old, none from the Permian or Triassic remains in place
for our examination.
However, there may be a way of reaching
back those quarter billion years to find traces of the "transient"
anaerobic resurgence.
Shallow seas (called epicontinental or
epeiric seas) lay on the continental margins just as they do today.
In fact, we have already noted one: the West Siberian Basin; there
were others. In some places, the sediments undoubtedly survive,
as sedimentary rock, possibly with a minimum of alteration. In
addition, the accreted pieces of ocean floor, as with the Japanese
boundary sections, may contain the necessary sedimentary evidence.
Even though many of the marine microorganisms lacked skeletons
(and Early Triassic deep marine environmental conditions would
probably have prevented their construction), biochemical traces
of their existence remain. These traces, especially when compared
to similar traces in pre-catastrophe Permian rocks, may be used
to determine the approximate makeup of Early Triassic marine populations.
Using the precision techniques of molecular
biology, such an analysis has already been done for a somewhat
more recent period in Earth's history. About 112 million years
ago, there was also a brief episode of oceanic anoxia, one of
several that have occurred during the Phanerozoic. This episode,
which happened in the early Aptian stage (stage is a geological
term referring to part of a period; in this case the period was
the Cretaceous), is referred to as "early Aptian oceanic
anoxic event 1b." Ocean sediments from that time contain
black shales, indicating anoxia, just as they do in the Early
Triassic. These black shales also record a negative carbon isotope
excursion (Kuypers, 2001).
Examined carefully using molecular biological
techniques, the black shales were found to contain large amounts
of distinct biochemicals (specifically "isoprenoidal tetraether
membrane lipids and free and macromolecularly bound isoprenoid
alkanes": Kuypers, 2001). These biochemical tracers are specific
to archaea; no other organisms contain them. They tell of an anoxic
ocean in which archaea are among the dominant organisms.
Similar analyses can be done on Early
Triassic black shales. If the suggestion of an archaeal (and anaerobic)
resurgence is correct, these analyses should show a high abundance
of those biochemicals specific to archaea in Early Triassic rocks,
and lesser amounts in rocks further from the Permian-Triassic
boundary.
As of January 2005, one major indicator
of a Permian-Triassic anaerobic resurgence has been found. Although
the evidence is not specifically of archaeal biomarkers, it does
provide conclusive evidence of an ocean which was euxinic at least
as high as the base of the photic zone (about 100 meters/yards).
Green sulfur bacteria, photosynthesizing anaerobes which live
at the oxic-anoxic interface of today's Black Sea, using the hydrogen
sulfide from below, produce their own distinctive biomarkers (isorenieratene
and chlorobactene). These biomarkers therefore serve as indicators
of photic zone euxinia, and those sedimentary rocks which contain
them speak unequivocally of euxinic (sulfidic) conditions. These
biomarkers have now been identified in Permian-Triassic boundary
rocks (Grice, 2005). Anaerobes had indeed taken over the depths
of the end-Permian and early Triassic oceans. (The question of
exactly how high into the shallow ocean euxinic conditions reached
was not determined in the Grice, 2005, study. But it is often
possible to make such determinations based on the particular rock
types in which the biomarkers are found: typically mudstones are
from deeper waters whereas marine sandstones are from shallow
waters closer to shore.)
End-Permian/Early Triassic
Extinction Mechanisms Summary Table
Deep Ocean
Suffocation (asphyxiation) by
anoxia; poisoning by hydrogen sulfide. Hydrogen sulfide also
produces "iron starvation" conditions. |
Shallow Ocean
Eutrophication (fertilization)
first depletes water of oxygen, killing by anoxia. With warming,
ocean stratifies, depriving phytoplankton of deep ocean nutrients.
Food web dissolves. Hypercapnia (too much CO¸2), hypermethia
(too much CH¸4), intermittent poisoning by hydrogen sulfide,
iron starvation, and acidification all take their toll. Ozone
depletion (?) increases mutation rate. |
Terrestrial Plants
Soil acidification; impairment of mycorrhizal (root symbiont)
fungi; extreme heat; inability to keep up with environmental
changes by moving with them. Ozone depletion (?) increases mutation
rate. |
Terrestrial Animals
Depletion of normal food sources; extreme heat; hypoxia, poisoning
by hydrogen sulfide (?). Ozone depletion (?) increases mutation
rate. |
Other Methane Catastrophes and Lesser
Hydrate Methane Releases
Although the end-Permian extinction is
recognized as the greatest extinction event of the Phanerozoic,
and the end-Cretaceous extinction is likely the second greatest,
the rank order of mass extinctions, according to magnitude, then
becomes somewhat murky. Paleontologists generally note five great
mass extinctions during the Phanerozoic (a sixth, at the end of
the Botoman Stage of the Middle Cambrian Period, identified by
Phil Signor, is often overlooked because of the unfamiliarity
of the affected organisms and its remoteness in time, at about
523 million years ago). These extinctions -- the end-Ordovician,
Late Devonian, end-Permian, end-Triassic, and end-Cretaceous,
are known as the Big Five (see diagrams and related text in the
Then section). In recent years, the Late Devonian mass extinction
has been somewhat downgraded, and is now probably ranked as the
least great of these extinction events (even so, it is hard to
regard an extinction event which kills off about 50% of the then-existing
genera as minor!). That leaves the end-Ordovician and the end-Triassic,
or Triassic-Jurassic, extinctions as roughly tied for third and
fourth, though some believe the end-Ordovician was the greater
(Kerr, 2001, Paring down the Big Five mass extinctions).
The Triassic-Jurassic extinction, which
marks the boundary between the Triassic and Jurassic Periods at
about 200 million years ago (older timescales put the boundary
somewhere between 213 to 205 million years ago), is nonetheless
a second mass extinction which may be traceable to a methane catastrophe.
This extinction event came after that of the end-Permian (250
million years ago) but well before that of the end-Cretaceous
(65 million years ago).
During the Triassic, many important new
groups of vertebrates had evolved: frogs and salamanders (amphibians),
lizards, crocodiles, and turtles (reptiles), and more advanced
but still relatively primitive mammals. Representatives of all
these groups made it through the end-Triassic extinction, as evidenced
by their presence in the modern world, as did pterosaurs, or flying
reptiles (which only made it as far as the end-Cretaceous).
But end-Triassic losses were staggering,
and included numerous groups of marine organisms. In all, about
80% of then-existing species met their demise (Sepkoski, 1996).
Most marine reptiles went extinct, though some of the dolphin-like
ichthyosaurs survived. Lots of ammonoids (distant relatives of
today's chambered nautilus) perished, along with many other mollusks
such as gastropods (snails) and bivalves (mollusks with two shells,
like today's clams). Possibly as few as 10% of bivalve species
made it into the Jurassic (Hallam, 1981, cited in Stanley, 1987).
Brachiopods and sponges were also hard hit, and conodonts (a formerly
enigmatic but ubiquitous group of organisms previously known only
by their tiny jawbones, now recognized as worm-like marine vertebrates)
expired forever, after more than 300 million years of existence
on the planet. Along the northern margin of the Tethys Ocean and
elsewhere, coral reefs disappeared (Stanley, 1987; Benton, 1990).
The reptile-like mammals which had survived
the end-Permian catastrophe and done relatively well during the
Triassic were decimated at its end, though enough survived (once
again) to ensure their descendants a place in the present world.
The reptiles which were to become dinosaurs fared even better.
Their Triassic ancestors, the thecodonts and their relatives,
had evolved a new posture, with legs upright under their bodies
(rather than the earlier sprawling-to-the-sides placement). This
upright placement allowed the thecodonts and their relatives to
move in a far less ungainly fashion than the reptiles they descended
from, and allowed for more rapid and efficient locomotion. Thecodonts
were one group of archosaurs, or "ruling lizards," whose
descendants were the dinosaurs. But the thecodonts themselves
were nonetheless wiped out at the end of the Triassic.
After the end-Triassic extinction, however,
the dinosaurs quickly took over the planet. The transition was
startlingly fast, a mere geological eyeblink. Within just 10,000
years of the Triassic-Jurassic boundary, older Triassic dinosaurs
had been replaced by much larger and more numerous dinosaur groups
(Olsen, 2002). They were to reign as the unchallenged masters
of the land for 135 million years, and their flying relatives,
the pterosaurs, were the largest flying creatures in Earth's history.
Despite the obliteration of both dinosaurs themselves and the
pterosaurs at the end of the Cretaceous, dinosaur descendants,
the birds, still rule the skies today.
What caused the great extinction at the
end of the Triassic? According to one scenario, it was the consequence
of an extraterrestrial impact, much as occurred at the end of
the Cretaceous. Indeed, in the sedimentary rocks that mark the
Triassic-Jurassic boundary, there is a spike in the amount of
iridium, one of the major pieces of evidence used to establish
an extraterrestrial impact as the cause of the end-Cretaceous
disaster. But the quantity of iridium is vastly lower, at only
285 ppt (parts per trillion: Olsen, 2002), compared to 9 ppb (parts
per billion) found in the sediments at the end of the Cretaceous
(Alvarez, 1997, p. 69). That's more than a thirty-fold difference.
It is certainly possible, of course,
that the end-Triassic sedimentary rocks that were sampled were
sufficiently far removed geographically from the point of impact
that the iridium concentrations were relatively small, and that
higher concentrations will eventually be discovered elsewhere.
But without additional impact-related confirming evidence, such
as the presence of shocked quartz (quartz that bears the mineralogical
signature of the force of impact), the impact theory remains conjectural.
The discoverers of the iridium anomaly
and the abrupt transition to larger dinosaurs at the beginning
of the Jurassic also note that the extinction seems to occur in
close temporal proximity to the eruption of the Central Atlantic
Magmatic Province (CAMP), the largest volcanic event of the Phanerozoic
(Olsen, 2002). They also note, however, that at least in eastern
North America, one of several major eruption sites for the CAMP
volcanics, the eruptions seem to have started some 20,000 years
after the end of the Triassic.
The Central Atlantic Magmatic Province
volcanics are found in continental areas on both sides of what
would become the Atlantic Ocean, in northwestern Africa and northeastern
South America. Like the Siberian Traps, they are the product of
fissure eruptions. Many of the fissures are quite impressive in
size, stretching for up to 300 kilometers (about 200 miles). Their
widths are 200 to 300 meters (yards) thick. Because these fissures
represent vertical cracks in the earth through which lava has
emerged, they are referred to by the geological term, dike. These
dikes typically occur in groups known as dike swarms, and are
found all along what would become the margin of the central Atlantic,
and up to 2000 kilometers (about 1200 miles) inland (Olsen, 2002).
They are related to the beginning of the opening of the Atlantic
Ocean.
The Central Atlantic Magmatic Province
represents the greatest known continental Large Igneous Province.
Up until just recently, that distinction belonged to the Siberian
Traps. But with CAMP volcanics having apparently covered more
than 7.5 million square kilometers (roughly 2.9 million square
miles: Marzoli, 1999), the Central Atlantic Magmatic Province
is significantly larger than the possible 3.9 million square kilometer
(about 1.5 million square mile) areal extent of the Siberian Traps
(Reichow, 2002), though it is possible that further investigations
may reveal that both of these are underestimates.
Investigations in Morocco, which was
approximately opposite present-day Nova Scotia and Newfoundland,
clearly show that the CAMP eruptions began before the end of the
Triassic, and peaked just about the time of the Triassic-Jurassic
boundary, or slightly thereafter (Marzoli, 2002). Thus, while
CAMP eruptions in eastern North America may have begun just after
the boundary, they were certainly already underway elsewhere.
In view of the modest size of the iridium anomaly at the boundary,
Central Atlantic Magmatic Province-related climate and biological
effects may indeed have been the cause of the end-Triassic extinctions
(Marzoli, 2002).
As with the Siberian Traps, these effects
would have included those produced by ash eruptions (transient
global cooling), ashfalls (oceanic fertilization; the clogging
of leaf stomata and the feeding apparatuses of aquatic filter-feeders)
and the release of volcanic gases such as carbon dioxide (global
warming), and including the poisonous halogens and hydrogen sulfide.
Perhaps most important, however, could have been the release of
methane from hydrate.
As at the Permian-Triassic and Paleocene-Eocene
boundaries, the Triassic-Jurassic boundary also records a major
negative carbon isotope excursion, here as much as 3.5 per
mil, recorded in the isotopes of both organic and inorganic compounds
(Beerling and Berner, 2002). Although volcanic outgassing of carbon
dioxide can account for a small portion of the isotope excursion,
it is quite inadequate (even with other possible consequences,
such as a global collapse of photosynthesis and weathering, considered)
to constitute the entire explanation. Only the rapid release of
about 5000 billion metric tons (Gt) of extremely isotopically
light carbon -- from hydrate methane -- could have provided the
needed jolt to lower the globally recorded carbon isotope signal
to its observed level. Interestingly, at least this quantity of
methane hydrate is assumed to have existed in the ocean sediments
of the "hothouse" conditions of the Triassic-Jurassic
world (Beerling and Berner, 2002).
It should be noted that the scientists
who trace the end-Triassic extinction to CAMP volcanics and the
consequent release of hydrate methane believe that the trigger
for that release was the global warming that attended the outgassing
of carbon dioxide from the volcanic eruptions (Beerling and Berner,
2002). However, it seems reasonable to assume that an extensive
system of magmatic sills formed in adjacent oceanic (and continental)
areas as a result of CAMP volcanism, and that those sills contributed
to the release of hydrate methane at the Triassic-Jurassic boundary,
in a similar fashion to the process that occurred at the Paleocene-Eocene
Thermal Maximum (Svensen, 2004), and presumably at the Permian-Triassic
boundary as well.
The relative sizes of the volcanism at
the Triassic-Jurassic and Permian-Triassic boundaries naturally
gives rise to a question:
Since the large igneous province that was emplaced at the Triassic-Jurassic
boundary (CAMP) seems to have been significantly larger than that
emplaced at the Permian-Triassic boundary (the Siberian Traps),
why then should the end-Permian mass extinction have apparently
been much greater than that of the end-Triassic, even though both
were quite disastrous?
The simple answer is that we do not know,
although it is possible to offer some possibilities.
Volcanic eruptions may differ from each
other in numerous ways. Among them are the type of eruption (submarine
or subaerial; stratovolcano, shield volcano, or fissure eruption);
the areal extent and the volume of the lava they extrude; the
temperature, composition and viscosity of that lava; the proportion
and amount of ash and volcanic gases; the force with which these
products are propelled from the eruption sites (and therefore
how much is injected higher into the atmosphere, how long it will
stay there, and over what area it will rain out); the duration
and size of each eruptive episode; the total length of the eruptive
sequence; and so on.
Most important, however, may be the location
of the eruption, or in the mantra of the retail trade, location,
location, location. Depending on where a large igneous province
erupts, it can melt any nearby sea and/or continental ice, affect
Earth's albedo (reflectivity), and thus its ability to absorb
solar radiation, and also alter ocean circulation. Near-polar
regions are considerably more vulnerable than those close to the
equator, partly because the distance around the globe at such
latitudes is much less than elsewhere, and limits the geographical
dispersion of ashfalls and volcanic gases. In addition, the presence
of methane hydrates in permafrost is far greater at higher latitudes
than lower ones, and the hydrates in marine sediments are much
closer to the surface. These differences may be quite sufficient
to explain why the impact of end-Permian volcanism was greater
than that of the end-Triassic. But the proximity to nearby ocean
regions, their depth and their connection to the world ocean are
probably also significant factors.
The enumeration of these possible differentiating
factors, of course, assumes that all other things were equal.
They may not have been. The quantity of methane hydrate must vary
considerably with the warming and cooling of the world's oceans.
Although the Triassic-Jurassic boundary period may have constituted
a "hothouse," the Permian-Triassic boundary period was
probably cooler. (For the past several tens of millions of years,
from well before the beginning of the Ice Age at about 2 1/2 million
years ago, the planet has been in period of significant cooling.)
Presumably, therefore, there were simply more methane hydrates
around for end-Permian volcanics to liberate.
Moreover, it does take long periods of
time -- millions of years -- for hydrates to accumulate in the
oceans. (This process has been ingeniously named the "clathrate
capacitor" [Dickens, 2003], for its similarity to the electrical
device known as the capacitor. A clathrate, you will recall, is
the icy lattice-like structure that makes up a hydrate; a capacitor
accumulates electrical charge and then releases it in a single
burst.) Slow leakage from the global methane hydrate reservoir,
therefore, or a recent discharge from that reservoir, will prevent
further discharges for some time.
There is a final difference between the
global changes that accompanied the Permian-Traissic mass extinction
and those which occurred at the Triassic-Jurassic: the presence
of a euxinic world ocean. Obviously there were specific reasons
why the Early Triassic world ocean became euxinic, and they probably
include the level to which ocean temperatures rose and/or oceanic
stagnation. Similar conditions do not sem to have been present
during the Early Jurassic. That is, at least as far as is known.
There apparently were several other methane-release
events during the Mesozoic Era (250 to 65 million years ago).
Most notable is the methane-release event of the Early Toarcian,
some 183 million years ago, which occurred at the Early-Middle
Jurassic Period boundary. As with the end of the Paleocene (LPTM)
and the end-Permian, this major methane release may have been
triggered by the eruption of a large igneous province (LIP), in
this case, the Karroo Igneous Province in South Africa (Hesselbo,
2000; Svensen, 2004). The Early Toarcian event is classified as
an oceanic anoxic event (OAE), because many of the indications
of deep ocean anoxia are present.
The Toarcian event displays the negative
carbon isotope excursion characteristic of methane releases, though
it was originally thought to have been a positive excursion. Newer
research on this event, however, indicates that the excursion
was indeed negative (Hesselbo, 2000), with its previous description
as positive presumably the result of reading of the recovery phase
as the onset of the excursion. A similar problem may be responsible
for a positive excursion recorded at the end of the Cenomanian
(about 90 million years ago), though as yet there is no evidence
to confirm that. Several oceanic anoxic events in the Aptian (about
116 to 112 million years ago) and at the Albian-Cenomanian boundary
(about 99 million years ago) (Gröcke, 2006), on the other
hand, are clearly accompanied by negative carbon excursions.
As measured in fossil wood and jet, a
form of coal occasionally used for jewelry because of its high
gloss, the excursion measures roughly 5 per mil. (The excursion
measures 4 to 7 per mil in organic matter; 2
to 5 per mil in marine carbonates; and the researchers use
2 to 3.5 per mil "as a basis for discussion."
In one rock section, the excursion is up to 9 per mil.)
Careful evaluation of the time extent of the excursion restricts
it largely to about 70,000 years. But a 2 per mil drop occurred
in less than five thousand years.
The isotopic excursion was also accompanied
by the formation of black shales, with an organic content sometimes
exceeding 10%. While the researchers note the similarity of this
excursion to that of the end-Permian, they dismiss the possibility
that the excursion could have been caused by the mechanism proposed
for the end-Permian by Knoll, 1996, oceanic overturn. Instead,
they attribute the carbon isotope drop to a "voluminous and
extremely rapid release of methane from gas hydrate contained
in marine continental-margin sediments," equivalent to 14
to 24% of today's methane hydrate reservoir (Hesselbo, 2000).
The latest paper on the Early Toarcian
event (Kemp, 2005) finds geological evidence for three negative
carbon isotope excursions, in quick succession over some 60,000
or so years, adding up to a total negative excursion of about
5 to 7 per mil. The second excursion occurs about 20,000 years
after the first, and the third rough;y 40,000 after the second.
This leads the paper's authors to tie the negative excursions
to the cyclic top-like wobbling ("precession") of the
Earth's axis (Kemp, 2005). Precessional cycles take about 20,000
years.
These cycles can have an effect on climate.
Were the Earth the same distance from the sun throughout the course
of its orbit, the precessional cycle would have no effect. But
Earth's orbit is not a circle around the sun, but rather an ellipse.
As the planet swings round the sun, it is sometimes closer to
the sun and sometimes further away. When closer, solar radiation
is more intense.
Consequently, when the planet is closer
to the sun during the northern hemisphere's winter (which occurs
when the north pole of Earth's axis is tipped away from the sun),
winter here is somewhat warmer than it would be otherwise. At
the same time, summer in the southern hemisphere, which occurs
during the northern hemisphere's winter, is somewhat cooler, because
that hemisphere receives less solar radiation. We are currently
at this point in the precessional cycle.
At the opposite point in the precessional
cycle, roughly 10,000 years in the past or future, Earth is closer
to the sun during the northern hemisphere's summer (which occurs
when the north pole of Earth's axis is tipped toward from the
sun), making summer warmer (and conversely for the southern hemisphere's
winter, which would be colder). During this part of the precessional
cycle, northern hemisphere winters are colder, and southern hemisphere
summers warmer.
The precessional cycle, therefore, alters
the timing of the seasons, and consequently shifts the seasons
from milder to more intense (with both colder winters and warmer
summers) and back again, over the course of about 20,000 years.
Such a shift could affect the warmth and circulation of the oceans,
particularly because the hemispheres differ considerably in their
distributions of ocean and land. (Today's southern hemisphere
actually has far more water, and the northern hemisphere the bulk
of the land. The distribution was much the same some 55 million
years ago, though there was somewhat more land area south of the
Equator than there is now.) Changes in the warmth and circulation
of the oceans, of course, could impact the stability of seafloor
hydrates.
But this precession-related variation
in itself would be insufficient to release seafloor methane. After
all, in the past 2.4 million years of the Ice Age, Earth has gone
through numerous precessional cycles without any major seafloor
methane release. For the precessional cycle to produce such a
release, Kemp and his co-authors indicate, the seafloor hydrates
must have been at threshold conditions: "We suggest that
the initiation of long-term global warming . . . associated with
the inception of the Karoo-Ferrar volcanic activity, when coupled
with solar insolation maxima [greatest solar radiation] controlled
by precession, was able to periodically exceed a climatic threshold
and thereby trigger the thermal dissociation of continental shelf
methane hydrate deposits" (Kemp, 2005).
Despite the authors' conviction that
the carbon isotope perturbation "was astronomically controlled"
(Kemp, 2005), however, there is reason to be skeptical of this
asserted "fact." Precessional cycle climate changes
are gradual, not abrupt, though it is certainly possible that
gradual change brought the climate to a "tipping point"
where abrupt methane releases could occur. More problematic is
the specific timing of the three negative carbon isotope excursions,
which have been labeled A, B, and C.
About 20,000 years, roughly equivalent
to one precessional cycle, elapse between the first two excursions
(A and B). But about 40,000 years -- roughly equivalent to two
precessional cycles -- elapse between the second and third excursions
(B and C). Thus, there is actually only one period between excursions
which approximates the precessional cycle, that between the first
and second excursions (A and B). For the next negative excursion,
the equivalent of roughly two precessional cycles must elapse.
There is no obvious reason for such a delay.
Moreover, just where one, based on the
precessional cycle theory, would expect third negative carbon
isotope excursion -- about 20,000 years after the second excursion
-- one finds a positive carbon isotope excursion of roughly the
same magnitude (about two per mil) as each of the three negative
excursions instead. Certainly, the purported astronomical control
of the negative excursions/hydrate releases requires additional
support, especially when random episodes of Karoo-Ferrar volcanism,
with the same timing, could have produced the same result.
The paper does note, however, that the
apparent methane hydrate release(s) were related to a sudden increase
in seawater temperatures, greatly increased global weathering
rates (presumably the consequence of more, more acidic, and more
intense weather events), and mass extinctions on land and in the
ocean (Kemp, 2005).
An additional hydrate methane release
event seems to have taken place later in the Jurassic, during
the Oxfordian Stage between about 159 and 154 million years ago.
Once again, a significant negative carbon isotopic excursion (at
least 2 per mil) is observed in both organic and carbonate
rock records. Once again, the hydrate methane release apparently
occurred during "greenhouse" conditions, further increasing
global temperatures (Padden, 2001). But in this case, the release
may not have been triggered by an episode of volcanism, but rather
by the slow movement of the tectonic plates, opening a marine
passage between the Americas, and allowing warm water from the
Tethys Ocean to pour into the cooler Panthalassa (Padden, 2001).
No significant biotic crisis seems to have attended this release
event.
A "striking but brief" negative
carbon isotope excursion (of 7 per mil, as measured in land
plant fossils) has been noted at the Middle Aptian Ocean Anoxic
Event (also referred to as the "Livello Selli" event),
during the Cretaceous Period, about 120 million years ago. The
magnitude of this excursion, once again, was found to be too great
to be explained by either volcanogenic carbon dioxide or by carbon
dioxide produced by a significant die-off of living things, leaving
hydrate methane as the only tenable cause. The drawdown of oceanic
oxygen in this event, moreover, seems attributable to the hydrate
methane release, which "could result in stratified oceanic
anoxia" (Jahrens and Arens, 1998).
There have been a number of relatively
recent methane-related warming events, but whether they can be
attributed to methane from hydrate or from wetlands is in dispute.
James Kennett and co-authors (2003) argue that the release of
seafloor methane has caused several of the warming episodes that
have punctuated the Ice Age, the time of intense cold that has
largely characterized Earth's climate for the past 2.4 to 1.8
million years.
(We are currently in one of the periodic
warming spells in that Ice Age. These warm periods have typically
lasted about 4000 to 6000 years; ours, known as the Holocene,
has lasted about 12,000 [White, 2004]. New ice cores from Antarctica
dating back as far as 740,000 years [EPICA, 2004] indicate that
if our current warming period is comparable to that of about 410,000
years ago, which it resembles, Earth might -- if the natural cycle
were followed -- be expected to stay free of major Northern Hemisphere
ice sheets for many additional thousands of years. Of course,
our active intervention in the climate cycle has been altering
and will continue to alter any natural patterns.)
Called the "Clathrate Gun"
hypothesis (again, clathrate is a general geological term for
gas trapped in a water ice lattice, but here specifically refers
to methane hydrate), the proposal ties high atmospheric levels
of methane to large submarine slumps. The proposal has received
support for Ice Age intervals when deglaciation was occurring,
though no link between slumping and atmospheric methane during
short-term warmer spells, at least over the most recent 45,000
years, has been established (Maslin, 2004).
Whether the methane came from marine
hydrates, however, has been challenged in a new and ingenious
study. While we have previously focused on negative carbon isotope
excursions as indicators of hydrate methane release, it is important
to remember that methane is composed of both carbon and hydrogen.
The new study (Sowers, 2006) looks at the source of the Ice Age
methane pulses, as determined by the isotopes of hydrogen.
Hydrogen has three isotopes, Hydrogen-1,
Hydrogen-2 (deuterium), and Hydrogen-3 (tritium). Hydrogen-1 has
just a single proton in its nucleus, but Hydrogen-2 has both a
proton and a neutron, and Hydrogen-3 has a proton and two neutrons.
Hydrogen-3 is radioactive, is found in tiny quantities, and does
not concern us here. Deuterium, however, is mixed in with "normal"
hydrogen (Hydrogen-1) in all the waters of the planet, and in
methane as well. The deuterium:hydrogen (D/H) ratio varies considerably
according to the source of the methane, and therefore allows for
the evaluation of the methane found in Greenland ice cores. A
relatively high ratio of deuterium to hydrogen is observed in
these cores, and is apparently traceable to its release from wetlands
rather than from marine hydrate (Sowers, 2006). Although the study
only looked at methane released back to about 40,000 years ago,
it suggests that the Clathrate Gun hypothesis may be in error.
CONTINUE TO NEXT SECTION (Part II: Now; The
methane catastrophe that awaits us tomorrow)
RETURN
TO CONTENTS






